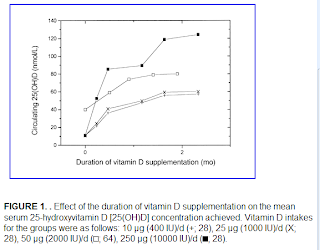
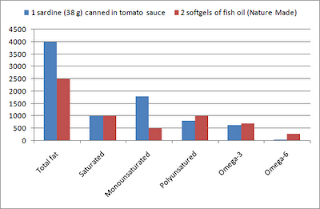
Charles Darwin, perhaps one of the greatest scholars of all time, thought about his theory of mutation, inheritance, and selection of biological traits for more than 20 years, and finally published it as a book in 1859. At that time, many animal breeders must have said something like this: “So what? We knew this already.”
In fact George Washington, who died in 1799 (many years beforeDarwin Pennsylvania
Washington
So, not only did animal breeders, like George Washington, had known about the principles of mutation, inheritance, and selection of biological traits; but they also had been putting that knowledge into practice for quite some time before Darwin’s famous book “The Origin of Species” was published.
Yet,Darwin Darwin
Recent research, for instance, suggests that “surprise” improves cognition. Let me illustrate this with a simple example. If you were studying a subject online that required memorization of key pieces of information (say, historical facts) and a surprise stimulus was “thrown” at you (say, a video clip of an attacking rattlesnake was shown on the screen), you would remember the key pieces of information (about historical facts) much better than if the surprise stimulus was not present!
The underlying Darwinian reason for this phenomenon is that it is adaptively advantageous for our brain to enhance our memory in dangerous situations (e.g., an attack by a poisonous snake), because that would help us avoid those situations in the future (Kock et al., 2008; references listed at the end of this post). Related mental mechanisms increased our ancestors’ chances of survival over many generations, and became embedded in our brain’s design.
Animal breeders knew that they could apply selection, via selective breeding, to any population of animals, and thus make certain traits evolve in a matter of a few dozen generations or less. This is known as artificial selection. Among those traits were metabolic traits. For example, a population of lambs may be bred to grow fatter on the same amount of food as leaner breeds.
Forced natural selection may have been imposed on some of our ancestors, as I argue in this post, leading metabolic traits to evolve in as little as 396 years, or even less, depending on the circumstances.
In a sense, forced selection would be a bit like artificial selection. If a group of our ancestors became geographically isolated from others, in an environment where only certain types of food were available, physiological and metabolic adaptations to those types of food might evolve. This is also true for the adoption of cultural practices; culture can also strongly influence evolution (see, e.g., McElreath & Boyd, 2007).
This is why it is arguably a good idea for people to look at their background (i.e., learn about their ancestors), because they may have inherited genes that predispose them to function better with certain types of diets and lifestyles. That can help them better tailor their diets to their genetic makeup, and also understand why certain diets work for some people but not for others. (This is essentially what medical doctors do, on a smaller time scale, when they take a patients' parents health history into consideration when dispensing medical advice.)
By ancestors I am not talking about Homo erectus here, but ancestors that lived 3,000; 1,000; or even 500 years ago. At times when medical care and other modern amenities were not available, and thus selection pressures were stronger. For example, if your no-so-distant ancestors have consumed plenty of dairy, chances are you are better adapted to consume dairy than people whose ancestors have not.
Very recent food inventions, like refined carbohydrates, refined sugars, and hydrogenated fats are too new to have influenced the genetic makeup of anybody living today. So, chances are, they are bad for the vast majority of us. (A small percentage of the population may not develop any hint of diseases of civilization after consuming them for years, but they are not going to be as healthy as they could be.) Other, not so recent, food inventions, such as olive oil, certain types of bread, certain types of dairy, may be better for some people than for others.
References:
Kock, N., Chatelain-Jardón, R., & Carmona, J. (2008). An experimental study of simulated web-based threats and their impact on knowledge communication effectiveness. IEEE Transactions on Professional Communication, 51(2), 183-197.
McElreath, R., & Boyd, R. (2007). Mathematical models of social evolution: A guide for the perplexed.Chicago , IL : The University of Chicago
In fact George Washington, who died in 1799 (many years before
So, not only did animal breeders, like George Washington, had known about the principles of mutation, inheritance, and selection of biological traits; but they also had been putting that knowledge into practice for quite some time before Darwin’s famous book “The Origin of Species” was published.
Yet,
Recent research, for instance, suggests that “surprise” improves cognition. Let me illustrate this with a simple example. If you were studying a subject online that required memorization of key pieces of information (say, historical facts) and a surprise stimulus was “thrown” at you (say, a video clip of an attacking rattlesnake was shown on the screen), you would remember the key pieces of information (about historical facts) much better than if the surprise stimulus was not present!
The underlying Darwinian reason for this phenomenon is that it is adaptively advantageous for our brain to enhance our memory in dangerous situations (e.g., an attack by a poisonous snake), because that would help us avoid those situations in the future (Kock et al., 2008; references listed at the end of this post). Related mental mechanisms increased our ancestors’ chances of survival over many generations, and became embedded in our brain’s design.
Animal breeders knew that they could apply selection, via selective breeding, to any population of animals, and thus make certain traits evolve in a matter of a few dozen generations or less. This is known as artificial selection. Among those traits were metabolic traits. For example, a population of lambs may be bred to grow fatter on the same amount of food as leaner breeds.
Forced natural selection may have been imposed on some of our ancestors, as I argue in this post, leading metabolic traits to evolve in as little as 396 years, or even less, depending on the circumstances.
In a sense, forced selection would be a bit like artificial selection. If a group of our ancestors became geographically isolated from others, in an environment where only certain types of food were available, physiological and metabolic adaptations to those types of food might evolve. This is also true for the adoption of cultural practices; culture can also strongly influence evolution (see, e.g., McElreath & Boyd, 2007).
This is why it is arguably a good idea for people to look at their background (i.e., learn about their ancestors), because they may have inherited genes that predispose them to function better with certain types of diets and lifestyles. That can help them better tailor their diets to their genetic makeup, and also understand why certain diets work for some people but not for others. (This is essentially what medical doctors do, on a smaller time scale, when they take a patients' parents health history into consideration when dispensing medical advice.)
By ancestors I am not talking about Homo erectus here, but ancestors that lived 3,000; 1,000; or even 500 years ago. At times when medical care and other modern amenities were not available, and thus selection pressures were stronger. For example, if your no-so-distant ancestors have consumed plenty of dairy, chances are you are better adapted to consume dairy than people whose ancestors have not.
Very recent food inventions, like refined carbohydrates, refined sugars, and hydrogenated fats are too new to have influenced the genetic makeup of anybody living today. So, chances are, they are bad for the vast majority of us. (A small percentage of the population may not develop any hint of diseases of civilization after consuming them for years, but they are not going to be as healthy as they could be.) Other, not so recent, food inventions, such as olive oil, certain types of bread, certain types of dairy, may be better for some people than for others.
References:
Kock, N., Chatelain-Jardón, R., & Carmona, J. (2008). An experimental study of simulated web-based threats and their impact on knowledge communication effectiveness. IEEE Transactions on Professional Communication, 51(2), 183-197.
McElreath, R., & Boyd, R. (2007). Mathematical models of social evolution: A guide for the perplexed.